- Home
- Education & Insights
- Search
- What Is Controlled Release? How it Works and Common Examples
What Is Controlled Release? How it Works and Common Examples

Table of contents
Controlled release (sometimes referred to as modified, sustained or extended release) is a pharmaceutical delivery technique that gradually dispenses active pharmaceutical ingredients (APIs) over a set timeframe. This method helps maintain stable drug concentrations in the bloodstream, enhancing treatment outcomes while lowering the risk of side effects, reducing dose frequency, and improving safety profile. This article will focus on oral drug delivery that makes up the largest volume of controlled release drugs on the market with 35% of all doses used in 2024.
Key Takeaways
-
Controlled release systems maintain consistent drug levels, improving efficacy and reducing side effects.
-
Oral controlled release formulations dominate the market, representing 35% of usage in 2024.
-
Controlled release mechanisms include diffusion, dissolution, osmosis, and ion-exchange, each tailored to specific drug properties and release profiles.
-
These systems enhance patient compliance and safety by reducing the dose frequency.
-
Reformulating drugs into controlled release versions can extend patent life and reduce long-term costs.
How Does Controlled Release Work?
Diffusion: The drug passes through a polymer barrier or matrix at a controlled rate.
- Reservoir devices: A drug core is coated with an insoluble polymer membrane. The drug diffuses through this membrane into the body.
- Hydrophilic matrices: A gel layer forms, allowing drug release via diffusion and erosion. The polymer’s viscosity (e.g., HPMC) is used to tailor the release profile.
How Hydrophilic Matrices Work
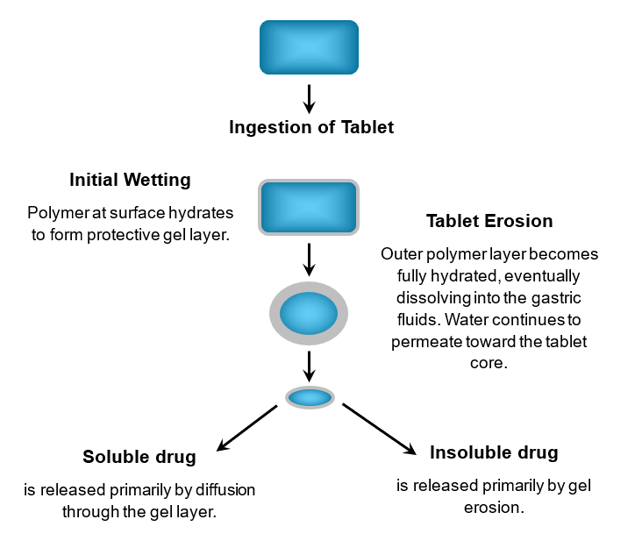
Dissolution: In this method, the drug's release is controlled by how quickly the tablet, capsule, or drug particles dissolve.
- Slow-dissolving coatings: Drug particles are covered with a material that dissolves slowly (e.g. ethyl cellulose), releasing the medication over time. By varying the coating's thickness, manufacturers can control the release rate.
- Dissolving matrix: The drug is embedded within a matrix that slowly erodes or dissolves in the body, releasing the API.
Osmosis: Often used in the form of push pull osmotic pump (PPOP) systems, these formulations deliver a constant, predictable drug release rate that is largely unaffected by conditions in the gastrointestinal (GI) tract. Such drug delivery is critical for APIs that are highly affected by GI conditions.
Essentially a PPOP will consist of the following:
- Drug (“pull”) Layer: which contains the API, designed for controlled release.
- Osmotic (“push”) Layer: which contains agents that create an osmotic gradient to drive drug release. Absorbs water, swelling the medication to “push” the drug out through the orifice. (e.g., Corelease™ OPL)
- Semipermeable Membrane: which allows water to enter and is critical for controlling the rate of the drug release. (e.g., Corelease™ CA).
- Orifice: a precisely drilled hole where the drug is released at a steady rate.
Ion-exchange: This mechanism is used for drugs that have a specific electrical charge. The drug is bound to a water-insoluble resin and as the medication moves through the GI tract, it exchanges with ions in the body's fluids, causing the drug to be released from the resin at a controlled rate, and often within a controlled range of pH.
Benefits of Controlled Release Medications
Maintain stable drug concentration: Unlike immediate-release medications, which create sharp peaks and troughs in drug levels in the bloodstream, controlled-release medications provide a consistent, uniform level of the drug over an extended period.
Reduced side effects: By avoiding the high concentration "peak" immediately following a dose, controlled-release formulations minimize the risk of dose-related adverse effects.
 Improved patient compliance: Controlled-release medications require less frequent dosing, meaning fewer tablets per day, making it easier for patients to adhere to their prescribed treatment regimen. This can be valuable for patients with chronic illnesses or those with difficulty swallowing.
Improved patient compliance: Controlled-release medications require less frequent dosing, meaning fewer tablets per day, making it easier for patients to adhere to their prescribed treatment regimen. This can be valuable for patients with chronic illnesses or those with difficulty swallowing.
Enhanced efficacy: For drugs with a short half-life, a slow, controlled release prolongs the duration of the drug's action, which improves the overall therapeutic benefit.
Protects sensitive drugs: Delayed-release formulations protect APIs from the acidic environment of the stomach, allowing them to pass into the small intestine, where they can be absorbed more effectively.
Reduced costs: While initial development may be more complex, the manufacturing of some controlled-release systems (i.e. matrix tablets) can be cost-effective. For manufacturers, reformulating an existing drug into a controlled-release version can also extend its patent protection and market life.
Types of Controlled Release Formulations
The following table provides an overview of various controlled release formulations, highlighting their mechanisms, typical applications, and key considerations.
|
Controlled Release Type |
Mechanism |
Advantages |
Example Drugs |
Clinical Use Case |
|
Delayed Release |
Uses pH-sensitive coatings or time-based mechanisms that release the drug after passing through the stomach or after a time delay. |
Protects drug from acidic stomach environment; reduces gastric irritation; allows for targeted intestinal release. |
Enteric-coated omeprazole |
Acid reflux treatment; protects drug from gastric acid. |
|
Extended Release (ER) |
Matrix, reservoir, or osmotic systems that slowly release drug over an extended period. |
Reduces dosing frequency; improves patient compliance; maintains therapeutic drug levels longer. |
Metformin ER, Wellbutrin XL |
Chronic conditions requiring long-term therapy, e.g., diabetes, depression. |
|
Sustained Release (SR) |
Similar to ER but may lack precise control of release rate; achieved through hydrophilic matrices or coated particles. |
Improves bioavailability; reduces fluctuations in plasma drug concentration. |
Theophylline SR |
Managing chronic asthma by maintaining therapeutic levels. |
|
Controlled Release (CR) |
Drug release rate is constant and predictable, achieved using advanced delivery systems like osmotic pumps. |
Maintains steady drug levels; minimizes side effects; optimizes therapeutic outcomes. |
OROS methylphenidate (Concerta) |
ADHD treatment with steady drug release over the day. |
|
Targeted Release |
Drug is activated by physiological conditions (pH, enzymes) or materials that release in specific organs. |
Enhances drug concentration at specific site; reduces systemic side effects. |
Mesalamine (colon-targeted) |
Treatment of ulcerative colitis by delivering drug directly to colon. |
|
Pulsatile Release |
Releases drugs in bursts, mimicking natural rhythms or timed to disease cycles. |
Synchronizes drug delivery with circadian rhythms; ideal for conditions with timed symptoms. |
Pulsincap systems; Propranolol CR |
Chronotherapy for hypertension, asthma, or arthritis. |
|
Floating Systems |
Use low-density materials to float on gastric fluids, prolonging gastric residence time. |
Useful for drugs absorbed in stomach; improves bioavailability for drugs unstable in intestine. |
Floating metformin |
Diabetes management with prolonged stomach absorption. |
|
Bio adhesive Systems |
Formulations adhere to mucosal tissues, enhancing contact time. |
Increased absorption; targeted delivery to oral, nasal, or gastrointestinal mucosa. |
Mucoadhesive buccal tablets of nitroglycerin |
Rapid relief of angina via buccal absorption. |
|
Nanoparticle-Based Release |
Drug encapsulated in nanoparticles; release governed by surface/size properties. |
Improved solubility; targeted cellular delivery; enhanced stability. |
Doxil (liposomal doxorubicin) |
Targeted cancer therapy with reduced cardiotoxicity. |
|
Swelling-Controlled Systems |
Hydrophilic polymers swell and gradually release the drug. |
Slow-release for highly soluble drugs; predictable kinetics. |
Hydrogel-based ibuprofen systems |
Pain relief with controlled release behavior. |
Extended Release (ER, XR, XL)
Extended release (ER) refers to any medication that is released over a longer period of time in the body, thereby maintaining a therapeutic dose for longer. Other terms for this form of modified release formulation include controlled release (CR), prolonged release, sustained release (SR) and long-acting (LA). Additionally, the names of ER marketed products often incorporate a suffix such as CR, XL, LA, SR, or XR.
Sustained Release (SR)
Sustained release (SR) formulations slowly release APIs to maintain consistent blood concentrations. It is typically used for chronic conditions requiring steady drug levels and offers longer duration of effect compared to immediate release.
Delayed Release (DR)
Ideal for drugs targeting the intestine as release is delayed to protects drugs from the high acid environment of the stomach (e.g., enteric-coated tablets). Learn more about enteric-coated tablets.
Common Applications of Controlled Release Drugs
Conditions commonly treated with controlled-release medications include pain management, chronic illnesses such as hypertension, diabetes, and neurological disorders, as well as various cancer treatments.
In pain management, these formulations provide consistent pain relief over extended periods, reducing the need for frequent dosing. For chronic conditions, controlled-release drugs help maintain stable therapeutic levels and improve patient adherence. In cancer therapy, they can deliver chemotherapy or supportive medications in a sustained and controlled manner, minimizing side effects while optimizing treatment effectiveness.
Controlled Release vs. Immediate Release
Immediate Release (IR) medications dissolve quickly, delivering fast relief—ideal for acute conditions like pain, allergies, or infections. However, they often cause sharp spikes and drops in drug levels, which can lead to side effects and require multiple doses per day.
Controlled Release (CR) formulations release the drug gradually over time, maintaining steady blood levels. This reduces side effects and supports once-daily dosing, making them well-suited for chronic conditions such as hypertension, diabetes, and depression.
Doctors may choose IR for rapid symptom control or when adjusting doses. CR is preferred for long-term treatment, especially for patients who benefit from simplified regimens or consistent drug exposure. For example, CR antidepressants often improve tolerability and reduce nausea compared to IR versions.
Patient lifestyle also influences the choice: CR offers convenience for busy schedules or cognitive challenges, while IR provides flexibility for variable dosing needs. Ultimately, the decision depends on the condition, drug characteristics, and individual patient needs.
Summary and Key Takeaways
Controlled release systems offer significant advantages in maintaining therapeutic drug levels, improving patient adherence, and minimizing side effects. By leveraging technologies like diffusion, dissolution, osmosis, and ion-exchange, these formulations are tailored for chronic and acute conditions alike.
They are especially valuable in managing diseases such as diabetes, hypertension, and cancer, where consistent drug exposure is critical. As pharmaceutical innovation continues, controlled release will remain central to improving healthcare outcomes.
Frequently Asked Questions
-
What is the difference between extended release and controlled release?
Extended-release (ER) is the broad term for a medication that prolongs the release of its API, while controlled-release (CR) is a more specific type of ER that delivers the drug at a constant, predetermined rate. In simple terms, all controlled-release medications are extended-release, but not all extended-release medications are controlled-release.
-
Can you cut a controlled release medication?
No, you shouldn’t break a controlled release capsule or tablet. Doing so can cause the medication to be released immediately, leading to a potentially dangerous overdose and loss of therapeutic effect.
-
Do controlled release medications work better than regular ones?
The type of release mechanism for medications does not mean one works better than the other; instead, one is more suitable than the other depending on the drug, the patient, and the medical condition being treated.
-
Are controlled release drugs more expensive?
Controlled release medications have clinically validated advantages over IR medications and are therefore typically more expensive per dose than immediate-release medications. However, the higher initial cost is offset by improved therapeutic effect, better patient compliance and reduced overall treatment costs.
%20(1).jpg)
.png)
