- Home
- Education & Insights
- Search
- 6 Steps to Ensure Robust Design & Performance of Hydrophilic Matrices
6 Steps to Ensure Robust Design & Performance of Hydrophilic Matrices

Table of contents
Quality by Design (QbD) is a systematic approach to pharmaceutical development. It means designing and developing formulations and manufacturing processes to ensure predefined product quality.1 In the case of hydrophilic matrix tablets, using hypromellose as the rate-controlling polymer, it is critical to consider the variability in properties of the polymer2 in addition to the variabilities in API properties and processing conditions.
Keep the following 6 steps in mind when implementing a QbD strategy to ensure the robust design and performance of your hydrophilic matrices.
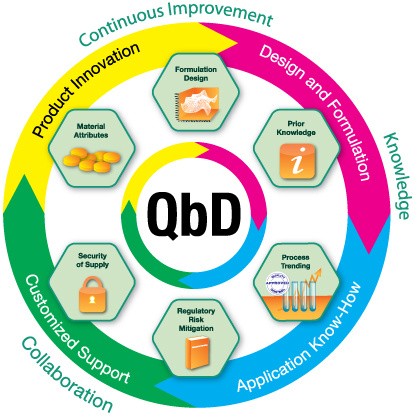
Optimize formulation design
Optimize formulation design
Tap into prior knowledge and expertise
Implement a manufacturing control strategy
Mitigate regulatory risks
Secure your supply chain
Suppliers should have a thorough business continuity plan and ideally multiple manufacturing sites to deliver security of supply, reduce operational risk, and provide consistent quality and availability of key materials globally.
Through the Controlled Release Alliance, Colorcon offers METHOCEL™ Premium Cellulose Ethers, the first choice in polymers for the formulation of hydrophilic matrix systems, providing a robust mechanism for the controlled release of drugs from oral solid dosage forms.
Connect with Colorcon to learn more about how you can incorporate a strategic QbD approach into your hydrophilic matrix formulation.
- Yu, L. X., Pharmaceutical Quality by Design: product and process development, understanding, and control. Pharmaceutical Research 2008:25(4): 781-791.
- Cabelka, T., Faham, A., Bernthal, H., Rajabi-Siahboomi, A., Application of Quality by Design (QbD) principles to the formulation of a hydrophilic matrix tablet of a high dose/high solubility drug. AAPS annual meeting and exposition, Los Angeles, CA 2009.

.png)

