Using Hypromellose (HPMC) in Matrix Tablets for Controlled Release
February 10, 2023
In the pharmaceutical industry, hypromellose (HPMC, METHOCEL™) serves as a filler/binder, as a polymer component of tablet coatings, and as a key excipient for controlled release. Hypromellose has been used in pharmaceutical tablets for over 60 years and is a well-accepted excipient for use in matrix tablets.
Many pharma companies use hypromellose for controlled release and especially for matrix tablet formulations. You may be wondering what your options are when it comes to HPMC products – especially if you are seeking something that you can market to your customers as label-friendly and sustainable. In this guide, we will talk about the key things you need to know about hypromellose.
What is Hypromellose?
Hypromellose is the now-accepted term for hydroxypropyl methylcellulose (HPMC), a polymer used as an excipient and for the controlled release of active ingredients (API) from oral matrix tablets.
HPMC polymers are semi-synthetic materials derived from cellulose, which is the most abundant polymer in nature. Some of the general properties of HPMC include:
- Soluble in cold water
- Insoluble in hot water
- Non-ionic
- Selective organo-solubility
- Reversible, inverse, thermal gelation
- Hydration and viscosity are relatively independent of pH
- Surface active
- Non-toxic
- Low taste and odor
- Enzyme resistant
- pH stable (2-13)
- Viscosifier, emulsifier, binder, rate-controlling agent, and film-former
What Are Hydrophilic Matrices?
A hydrophilic matrix tablet is a type of dosage form that makes it possible to control the release of a drug from a tablet over an extended time.
A hydrophilic matrix dosage form is:
- Relatively straightforward
- Requires standard tableting equipment
- Resistant to drug dumping
- Is not hindered by tablet hardness or compression force
- Can modify release kinetics at the excipients/polymer level
Use of hypromellose in hydrophilic matrices has broad regulatory approval, is easy to use, has an excellent safety record, and has been extensively studied. This makes HPMC an excellent choice for pharmaceutical companies to develop and manufacture controlled release tablets.
Factors Influencing Modified Drug Release in Tablets
When designing tablets for modified release, there are two main factors for consideration: formulation and process. There are sub-factors you should also consider when deciding on the formulation and the release profile for your final drug product.
Formulation
Key sub-factors to consider for early-stage development:
- Polymer (substitution type, viscosity, concentration, and particle size)
- Drug (particle size and solubility)
- Filler (solubility and concentration)
- Other excipients (stabilizers and buffering agents)
Process
These categories have to do with how the drug is produced:
- Method of manufacture
- Tablet size and shape
- Compression force
- pH environment
- Film coating
How Matrix Tablets Work
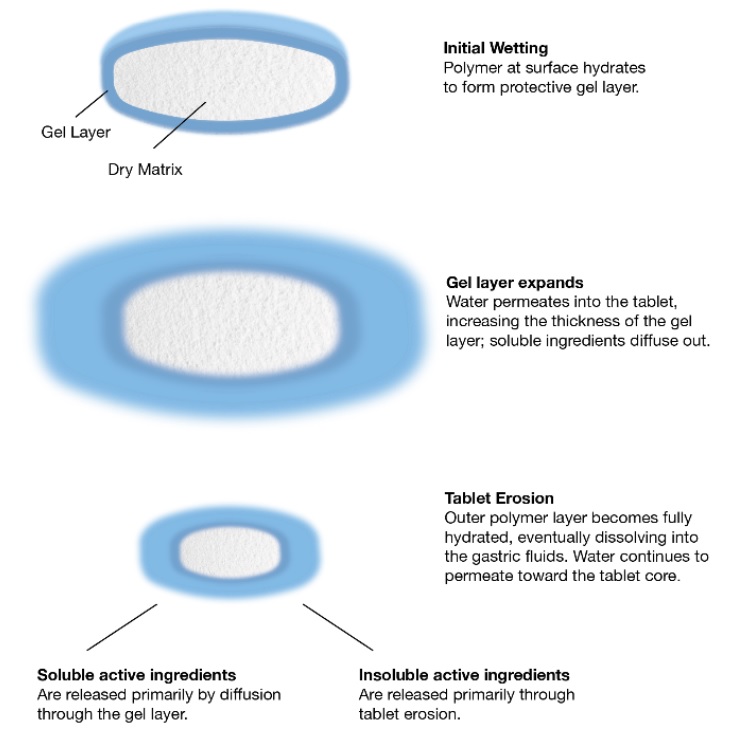
Hydrophilic matrices enable the controlled release of drug via the gel layer through diffusion (soluble active ingredients) and erosion (insoluble active ingredients) – with the viscosity of the polymer having a big impact on the release profile. HPMC allows pharma companies to modify the release profiles of their drugs using matrix tablet technology, providing more effective dosing and better patient compliance through reduced tablet burden. After all, once-a-day medicine is better than multiple tablets several times a day.
Technologies for Controlled Release
While HPMC (METHOCEL™) is a well-known and accepted polymer for controlled release, there are alternative technologies that may offer the prospect for narrowing the release profile or providing intellectual property opportunities.
Sustained release technologies from Colorcon use unique combinations of polymers to encapsulate active drug compounds and control release of the drug at varying rates. Through advanced technology, our sustained release products simplify the development and production process for tablets and capsules while providing consistent rates of drug release.
Colorcon offers advanced film coating systems, such as Surelease and Opadry EC for sustained release (also referred to as controlled release) dosage forms complemented by our value-added services, which support all phases of product design and development.
If you are interested in learning more contact us today for a consultation.
