Designed for use across food, medical and pharmaceutical applications, Colorcon No-Tox® printing inks and coatings are backed by 60+ years of experience, unmatched technical service and written FDA or EU compliance assurance.
Printing Inks with a Peace-of-Mind Guarantee

Non-Toxic Is Just the Beginning
Customers in 50+ countries rely on Colorcon No-Tox® inks and coatings, and for good reason: beyond guaranteed adherence to applicable regulatory requirements, No-Tox® delivers proven safety and performance across high-stakes products and use cases.

Food Contact Packaging
Manufactured in an ISO 9001 certified current Good Manufacturing Practices (cGMP) facility, No-Tox® inks satisfy stringent FDA or EU requirements for direct or indirect food contact across a wide range of applications: from coupons to containers, and foils to food tray liners. Overprint varnishes and coatings take it even further, delivering the exact substrate properties that packaging demands.
Medical Device Printing & Packaging
Tailored to meet FDA and EU requirements for devices and related packaging, No-Tox® ink solutions provide durable, clear markings for identification and branding, plus compatibility with sterilization. Even more, No-Tox® medical device dye solutions employ the standardized test method for proactive detection of defects in medical device package seals.
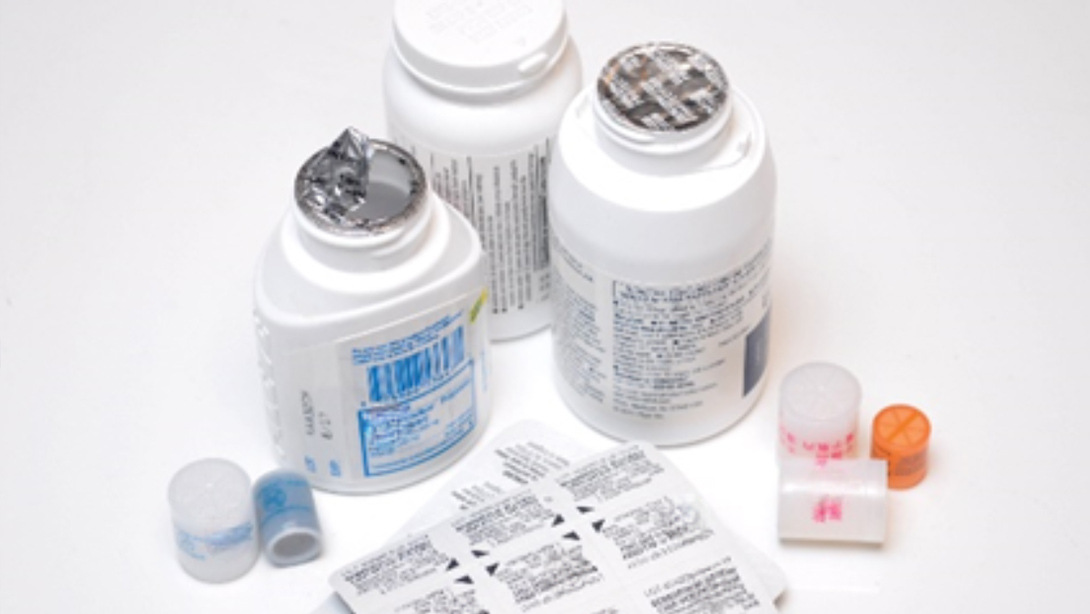
Pharmaceutical Printing Inks
Whether printing on drug packaging or the products themselves, No-Tox® specialized inks deliver safety, clarity and durability in any critical healthcare application. No-Tox® inks are manufactured in an FDA-registered facility compliant with Current Good Manufacturing Practice (cGMP) guidelines and free of compromising contaminants.

Specialty & Cosmetic Products
Merging regulatory compliance and innovative formulations, No-Tox® inks and adhesives ensure the highest degree of safety, quality and performance available for face paints, temporary tattoos and more. Ideal for applications where non-toxicity, water solubility, skin safety and/or odorless qualities are non-negotiable.
Product Library
Food Contact Packaging
Medical Device Printing & Packaging
Pharmaceutical Printing Inks
Specialty & Cosmetic Products
Place an Order
To place an order or for regulatory information, please visit My Colorcon.