

Beyond Keeping Up Appearances
When it comes to solid oral dosage form design, questions about film coating pros and cons are being asked by both marketers and regulatory bodies. Both groups are seeking answers that address growing concerns on how product design decisions help mitigate medication errors, target patient compliance, or compete with a growing number of generic and OTC options.
In readiness for these questions, today’s formulators need to consider film coating design options earlier in product development. Stephanie Sutton, Editor of The Medicine Maker, recently sat down with Colorcon regulatory and tablet design experts to discuss their views and bust some popular myths about the hurdles of coating.
To aid in the development of your own best practice, read a copy of the complete article here.
In-Use Protection for Moisture-Sensitive Dosage Forms
The drug combination of amoxicillin and clavulanic acid is recognized as being sensitive to moisture, demanding low humidity manufacturing conditions and moisture barrier packaging. This commercially marketed product is commonly coated with standard hypromellose film coating which is not specifically formulated for moisture protection.
In a two-part case study, Colorcon examined the stability of amoxicillin/clavulanic acid with uncoated and film coated tablets, including an hypromellose coating and an Opadry®amb II high performance moisture barrier film coating in a variety of packaging formats with room temperature conditions (in-use) and accelerated stability conditions.
In both cases, the data clearly demonstrates that Opadry amb II better maintains the stability of the amoxicillin and, more notably, the clavulanic acid in all packaging formats tested. This stability improvement ensures product quality and helps to maintain in-use product integrity.
Read more: Colorcon Case Study
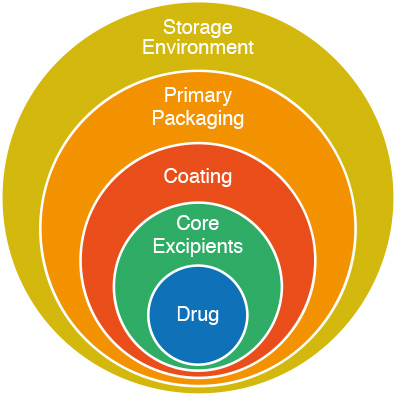
New Film Coating to Modulate Drug Release

Colorcon is delighted to introduce another innovative film coating system, Opadry® EC, ethylcellulose organic coating system, for the modulation of drug release from multiparticulate oral dosages.
As the market continues to grow for multiparticulate dosage forms, especially for geriatric and pediatric populations, Opadry® EC provides a fully formulated solvent coating option that enables a range of release profiles for these speciality dosage forms.
The base polymer for this system, ethylcellulose, is widely used in the pharmaceutical industry as an insoluble barrier membrane coating. This coating provides extended release profiles and taste-mask options for granules and orally-disintegrating tablet (ODT) dosages.
A Colorcon Study, Effect of Coating Weight Gain and Pore-Former Level on Drug Release with a Fully Formulated Ethylcellulose Barrier Membrane Coating System, presented at 2015 AAPS Annual Conference shows how Opadry EC enabled a range of release profiles to be obtained for multiparticulates. By adjusting the pore-former content and the applied coating weight gain to modify the barrier membrane porosity and thickness, a range of release profiles are possible. These results indicated that Opadry EC offers fast formulation screening during development and enables the flexibility to tailor release profiles for drugs with different solubility.
As a ready-to-use system, Opadry EC means cost effective and consistent coating performance, and helps to reduce potential errors that may occur during raw material handling and manufacturing.
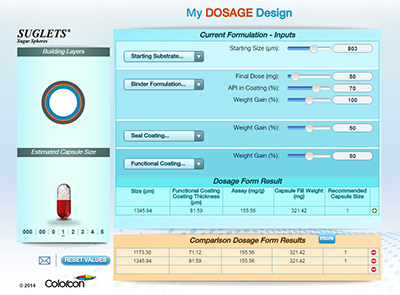
New Dosage Design Tool - When Size Matters
A first step of multiparticulate dosage design is selecting the inert starting substrate, setting the foundation for each following stage of development. While it's common to select smaller beads for higher dose products, this is not always the best practice. Very small substrates or beads can pose challenges including high drug layering weight gains, increased likelihood of agglomeration, low spray rates, long process times, and potential batch failures.

This photograph shows the fill volume for 400 mg of various sizes of Suglets® sugar spheres (beads) into size 3 capsules. The fill volumes are very similar for three different sized beads, driven by their packing density. The benefits of using a larger bead size, such as a 20/25 or 18/20 mesh Suglet, can outweigh the process challenges of working with very small beads (such as 45/60 mesh), while only minimally impacting capsule fill volume.
Colorcon has developed a tool to predict the effects of dosage, bead size, and film thickness on your overall capsule fill. Talk to your local Colorcon representative to see how My Dosage Design can help you design your next capsule project.

Suglets® Sugar Spheres Boosted by Recent Acquisition

Colorcon’s recent acquisition of Paulaur’s sugar spheres products for pharmaceutical use complements Colorcon’s Suglets® Sugar Spheres product line and enhances our position as a leading global supplier of sugar spheres for the development of multiparticulate dosages.
Colorcon will transition customers from Paulaur, and their distributors, to provide direct Colorcon sales and customer service and convert customers to Suglets® sugar spheres manufactured by Colorcon’s facilities located in Stoughton, Wisconsin, USA and Bazainville, France, where there is ongoing expansion of Colorcon’s sugar sphere manufacturing capacity.
We are excited about this expansion of our excipients business and the unrivaled support we can bring to meet the increasing demand for multiparticulate applications around the world.
Read more Colorcon-Paulaur Press Release
New Technical Services Facility in Bogota, Colombia
Designed to support market growth and customers across the Andean countries, Central America and the Caribbean, Colorcon’s new services laboratory in Bogota, Colombia officially opened its doors on March 2, 2016. Press release.
The facility has internationally recognized certification for the containment and safe handling of Level II and Level IV active pharmaceutical ingredients (APIs), and will provide customers with faster turnaround on technical services and increased capacity to handle more projects.
Outfitted with new equipment, and its location close to transportation hubs, the Bogota site has increased our ability to offer regional pharmaceutical companies direct access to an increased number of Colorcon Coating and Formulation educational programs in 2016.

| Now open for registration | |
|---|---|
| July 18-19 | Coating School |
| September 6-7 | Formulation School | Matrices |
| October 27-28 | Coating School |
Local Production Coming Soon to Brazil

Colorcon is pleased to announce the addition of its seventh Film Coating Manufacturing facility in Indaiatuba, Brazil. Equivalent in all aspects to our other film coating manufacturing sites worldwide, the Brazil facility will produce GMP film coating products for the pharmaceutical industry in South America. We are shortening lead times, reducing the risk of importation delays and further securing the supply chain for our customers. Press Release.

Vietnam City Seminar
Colorcon proudly sponsored its fifth Vietnam City Seminar, “SPEEDING HIGH-QUALITY GENERICS DEVELOPMENT”, attended by representatives from pharmaceutical companies located in South and Central Vietnam. The one-day program included case studies and topics selected to help the audience gain efficiency and reduce costs in the development of solid oral dosages.
Speakers, pictured from left to right: Mr. Frank Zhao, Dow, China. Mr. Nha Huynh, Colorcon, Vietnam. Ms. Rizky Aryanti, Dow, Indonesia. Ms. Truc Vo, Colorcon, Vietnam. Ms. Cathy Wang, Colorcon, China. Mrs. Fu YanHong, Dow, China. Mr. David Wei, Colorcon, Singapore. Dr. Gao Hao, Dow, China. Mr. Glenn Russell, Colorcon, Singapore.


