

Taste-Masking: Overcoming Effects of API Morphology
For more than twenty years, Surelease® and Opadry® have been used in combination to provide a taste-mask coating option resulting in a release profile with a slight initial delay, while maintaining the monograph criteria for an immediate release dosage form. However, the coating formulation alone does not dictate the efficacy of the coating. One critical parameter is the properties of the substrate that may impact the coating quality, process efficiency, and in turn taste-masking functionality.
In our latest technical bulletin on taste-masking, we highlight the impact of substrate morphology through a case study of two different commercially available grades of acetaminophen (APAP) coated with Surelease:Opadry combinations. The two particle grades vary in particle size, distribution and morphology, and the difference in release performance is significant. When developing a taste-masked dosage form, the formulator must consider the particle properties of the substrate to obtain the most robust product. Read the Technical Bulletin.
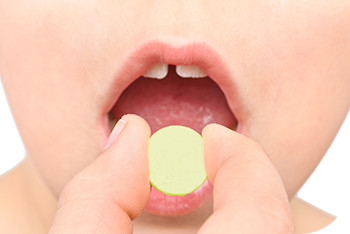
Colorcon is further expanding its taste-masking product portfolio with Kollicoat® SmartSeal, from BASF, considered a best-in-class reverse enteric polymer. Through collaboration with BASF, Colorcon is developing fully formulated pH-dependent coating systems based on Kollicoat® Smartseal, which are insoluble at the relatively neutral pH of the mouth and become soluble once in the lower pH of the stomach. This expands Colorcon’s taste-masking product portfolio, and allows us to better serve the industry in providing solutions to improve adherence, particularly in the more challenging paediatric and geriatric spaces.
Kollicoat® Smartseal is a registered trademark of BASF SE.

Continuous Coating Process: Study of Transit Times and Coating Uniformity

Continuous coating is rapidly becoming more than just a technology for a few companies with high volume products. At the AAPS 50th Arden Conference on Continuous Manufacturing (March 2015, Baltimore, MD) speakers from many of the largest global pharmaceutical companies and the FDA spoke about the need for a paradigm shift in pharmaceutical manufacturing.
The benefits and challenges of continuous manufacturing were highlighted and various key initiatives supporting this bold new shift in the manufacture of solid oral dosage forms were presented.
Colorcon was also on hand, presenting a poster: Evaluation of Film Coating Weight Uniformity, Tablet Progression and Tablet Transit Times in a High Throughput Continuous Coating Process. This work compared the coating uniformity values of a typical production-scale batch coating process with the continuous process. Through studies, we also demonstrated the utility of higher productivity coating systems, greater than 20% solids, in further maximizing the capacity of this type of coating equipment.
To access a secured copy of the poster, click here.
Improving the Physical Stability of Soft Gelatin Capsules by Film Coating
Physical stability of some soft gelatin capsules stored at high humidity and elevated temperature can be a concern, and agglomeration is often observed. Catalent Pharma Solutions recently published a study to demonstrate how the physical stability of soft gelatin capsules can be improved by film coating.
The study confirmed that using either Opadry® II or Opadry® 200 film coating formulations, resulted in better protection when compared to an EUDRAGIT® EPO formulation for soft gelatin capsules under accelerated storage conditions. The study also concludes that coating formulation and coating level play a critical role in physical stability of softgels.
EUDRAGIT® is a registered trademark of Evonik Röhm GMBH, Dramstadt, Germany.
 To access the full article, click here.
To access the full article, click here.
Introducing METHOCEL™ K200M Premium
and Premium CR

The Global Controlled Release Alliance; Colorcon & Dow Pharma & Food Solutions, is pleased to announce the addition of METHOCEL™ K200M Premium Cellulose Ethers and K200M Premium CR for use in extended release matrix formulations.
METHOCEL™ K200M offers the same quality control of critical material attributes associated with the established high viscosity METHOCEL™ polymers, available through the CR Alliance.
This expansion of the METHOCEL™ product line offers formulators additional tools to extend the release of very soluble drugs and to optimize finished tablet weight of matrix tablets.
For more information contact Colorcon Technical Service.
Colorcon Supports Customers Implementation of ICH Q3D – Elemental Impurities
The International Conference on Harmonization (ICH) and the United States Pharmacopeia (USP) have been developing guidelines and general chapters related to Elemental Impurities in drug products for many years. The ICH Q3D Step 4 guideline was published in December, 2014; these requirements will now be implemented in each region using the local regulations.
ICH Q3D applies to finished human drug products and does not specifically apply to components (i.e. drug substances/excipients). ICH Q3D emphasizes the use of risk assessment as opposed to testing. The ICH Q3D requirements of Permissible Daily Exposure Limits (PDEs) for finished drug products will become a regulatory requirement in the US and EU in June 2016 for new drug products and for existing drug products in January 2018.
Colorcon is performing internal risk assessments and investigative testing on our excipient and film coating products to obtain typical data that can assist customers in their risk assessments. Colorcon does not intend to establish specifications for elemental impurities except where required in a pharmacopeial monograph. Representative Colorcon products will be assessed and data is being generated on appropriate model products to provide some helpful information to customers. This information will be provided, upon request, to customers as part of our Product Regulatory Datasheets.
Request Information
Colorcon Continues Investment in China – Helping Accelerate Formulation Development

Colorcon officially opened a new technical service laboratory in Beijing, China on June 25, 2015. Located in the Beijing Yizhuang Biomedical Park, this technical centre is in a unique position to work closely with the pharmaceutical research community in the North China region on developing immediate and modified release solid oral dose formulations.
The Beijing lab is Colorcon’s 19th Technical Service facility, which are located strategically close to customer hubs around the world, and is the second Colorcon laboratory in China.
Simon Tasker, Managing Director, Colorcon NESEA, comments, “Investment in the Beijing facility reflects our view of the strategic significance of Northern China from a long-term growth perspective. It reinforces our commitment to providing best-in-class products and services through a network of laboratories positioned close to our customers. This service model approach is the unique value of Colorcon.”
Press Release
Colorcon’s UK Site Adds EXCiPACT™ Certification to Support Pharmaceutical Customers to Reduce Growing Regulatory Requirements
Colorcon is inspected and meets the requirements of appropriate licensing bodies around the world. In China, Japan and India, Colorcon operates under full pharmaceutical manufacturing license, and is inspected and approved according to pharmaceutical GMP”. In addition, the four manufacturing facilities in North America, along with the Bazainville, France site, meet the requirements of ISO 9001, which continues to be an industry recognised quality management standard.
For over 20 years, the Quality Management System (QMS) used by Colorcon Limited has been independently certified as meeting the requirements of ISO9001. EXCiPACT™ is an additional certification scheme for pharmaceutical excipients that demonstrates to customers our continued commitment to quality and managing risk on their behalf.

ISO9001 certified for over 20 years and a founding member of the International Pharmaceutical Excipient Council (IPEC), Colorcon Ltd, has been awarded EXCiPACT™ certification for its long established excipient manufacturing site in Dartford, UK.
Colorcon views EXCiPACT™ as a complementary element of our pledge to continuous improvement and our already established quality systems that help our customers reduce total cost of ownership.
According to Kevin McGlue, Colorcon Director of Global Quality Assurance and IPEC member “Colorcon has aligned its Quality Management System (QMS) with the Joint IPEC-PQG Good Manufacturing Practices Guide for Pharmaceutical Excipients. Colorcon Ltd. has also undergone regular voluntary GMP inspections with the UK Medicines and Healthcare Products Regulatory Agency (MHRA), resulting in confirmation of compliance with the IPEC-PQG Guide.
Colorcon Receives PRÊMIO SINDUSFARMA DE QUALIDADE Award

Fernanda Fugii, Regional Quality Assurance Manager
Camila Martins, Quality Assurance Manager
Celina Yassui, Quality Assurance Analyst
We are proud to announce that Colorcon, once again, received the Sindusfarma award for the best supplier of imported pharmaceutical raw materials during the 2015 FCE Exhibition held in Sao Paulo, Brazil.
As a result of voting by Quality and Purchasing Managers, Sindusfarma, a leading Brazilian pharmaceutical industry association, conducts a quality audit of the three finalists for this award. Only one of the three companies receives the final award, based on that inspection.
Colorcon’s receipt of the award was made possible through the great teamwork conducted by all Colorcon’s employees in Brazil who continuously strive to exceed our customer’s expectations for high quality products and technical services.
We give special thanks to all the customers who elected Colorcon Brazil as your supplier of choice. PRÊMIO SINDUSFARMA DE QUALIDADE – A great recognition from you.




