3 Tips to Boost Patient Compliance with Tablet Design
Purposeful, careful design of tablets is a key driver for consumer preference – but not all in the industry are taking advantage of this branding opportunity.
Each year, the U.S Food and Drug Administration (FDA) approves new solid oral dose prescription medications – and according to industry data (Pharma Circle) around 25% of those dosages are still plain white tablets (although the numbers are declining).
Add these new drugs to products already on the market, and it quickly becomes clear why the pharmaceutical industry needs to pay greater attention to tablet design. Here, we will talk about how a focus on safety in the design of tablets help prevent manufacturing and packing mix-ups, increase patient compliance and satisfaction, and boost your market share as a result.
Smart Tablet Design is in Everyone’s Best Interest
It’s important to understand why smart design of tablets and solid oral dosage drugs is important for everyone – not just pharma companies, but consumers as well.
In 2017, the FDA removed nearly 2,000 drugs and biologics from public use — recalls that hurt sales, damaged the important relationships companies built with customers, and disrupted both supply and supply chains. Poor design also can prompt the FDA to reject regulatory submissions.
And there’s a heartbreaking human cost to poor tablet and capsule design. A landmark report from the Institute of Medicine examining human error as a leading cause of death found that some 7,000 people die each year (USA) because of medication mistakes made within the health-care system.
Adding to this tragic loss of life, the World Health Organization (WHO) reports that prescription drug errors cost US$300 billion annually in avoidable medical waste.
In recent years, both the FDA and European Medicines Agency (EMA) issued guidance encouraging patient perspectives to be considered when designing tablets and capsules, a practice the pharmaceutical industry now refers to as “Safety by Design.”
What Is Patient Compliance?
The term "patient compliance" refers to the degree that a patient follows medical advice and takes a full course of prescribed treatment. Good patient compliance means people consume the medication as directed, which leads to better outcomes for the patient and a more positive experience with the drug product. Poor patient compliance results in the opposite and often leads to poor patient outcomes.
Tablets created with safety by design in mind have higher patient compliance, which is why it is important for pharmaceutical companies to make this a key consideration when designing their tablets.
3 Tips for Better Patient Compliance Using Safety by Design for Tablets
A focus on safety in tablet design can boost patient compliance. Here are three tips to put this into practice.
Tip 1: Avoid Plain White Tablets
As our population ages, polypharmacy is becoming more commonplace. Currently, about 15% of the U.S. population now takes five or more prescription medications.
So now more than ever, it is increasingly important to create easily distinguished tablets. Similar looking tablets — especially plain white ones — can be readily confused by patients, pharmacists, caregivers and even manufacturers.
One recent example involving a company’s voluntary nationwide recall of generic phenobarbital tablets occurred when it was discovered that a bottle containing 30 mg tablets were mistakenly labeled as 15 mg tablets.


C.O. Truxton, Inc. voluntarily recalled white phenobarbital tablets (30 and 15 mg strength). The 15 mg tablet is debossed with “West-ward 445” on one side and blank on the reverse side; the 30 mg tablet is debossed with “west-ward 450” on one side and scored on the reverse side.
By changing the color and/or shape of the tablet, mislabeling could have been detected prior to distribution.
Tip 2: Differentiate Dosage Strengths
When drug manufacturers are producing multiple dosage strengths, regulatory agencies will look to ensure the color differentiation is clear to medical personnel and lay persons alike.
Recently, a major pharmaceutical manufacturer requested Colorcon’s help with tablet design after their initial NDA submission to the FDA was rejected; regulators said that the doses were too similar in appearance. Judge for yourself.
Rejected submission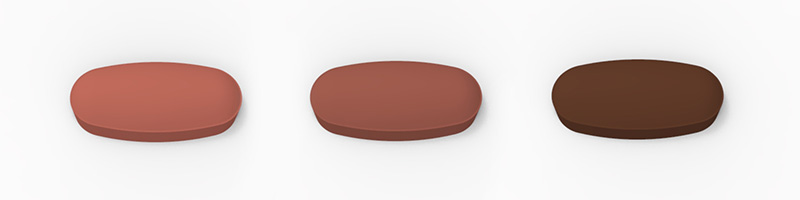
Approved submission

Tip 3: Choose Colors for Recognition and Branding
Pharmaceutical companies have a wide spectrum of opportunities to add color to choose from. However, the pigments, ingredients, and regulatory requirements for use will define what is approvable for your final use in either pharmaceutical or nutritional products.
For example, our online Color Selector, available to registered Colorcon website users, provides a great starting point to explore color choices. Our specialists work with companies to confirm the right coating system to meet their functional needs and check the acceptability for your application and target market.
Want to learn more? Connect with Colorcon to determine the BEST way to differentiate your product and satisfy regulatory requirements.
