Fluid Bed Best Practices for Multiparticulate Formulations – Part 2
Process Control and Product Quality using Computational Design and Process Analytical Technology (PAT)
Multiparticulate dosage forms are complex and the application of coatings to extend drug release can present challenges. Substrate properties, coating formulation, equipment and process conditions are all considerations that affect final drug release. Based on Colorcon’s experience we have developed best practice guidelines that ensure speedy development and a trouble-free lifecycle for your commercial multiparticulate dosage product.
This second installment covers the importance of process monitoring when applying a coating to multiparticulates for extended release performance.
Real-Time Process Monitoring to Track Process Control and Predict Performance
Multiparticulate (MP) dosage forms provide formulation and process opportunities to modulate drug release characteristics and target specific release sites within the GI tract. Fluid bed bottom-spray (Wurster) coating is routinely used to apply successive coating layers in the development and manufacture of these multiparticulate formulations.
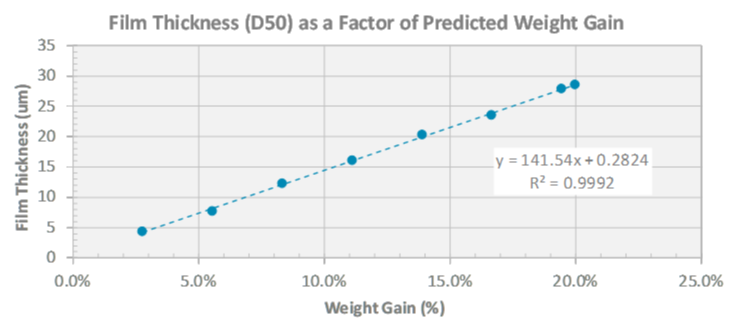
During development, a key consideration is the amount of coating applied to achieve the target release profile. Typically, the quantity of coating is determined by the application of increasing % weight gain until the desired release is obtained. While this approach has been successful, it’s time-consuming, ignores raw material variability and lacks the insight necessary to understand a coating process and achieve repeatability. In Part 1 of this series, we introduced the importance of the surface area to film thickness ratio (SA/FT) to overcoming these challenges to design a robust multiparticulate formulation.
Process tracking of a fluid bed process, in-line or at-line, from development to manufacturing promotes a greater level of understanding, reduces risk, enhances control and the ability to build quality into a product.
The Importance of Innovative Development and PAT in Fluid Bed Coating
Process analytical technology (PAT) is broadly considered to include any chemical, physical, and mathematical analysis conducted in an integrated manner. Incorporating this type of analysis within a manufacturing process allows timely monitoring and control of the critical processes and quality attributes that can help ensure finished product performance.
Dynamic image analysis is one PAT technique (Eyecon2™) that has been successfully integrated into the fluid bed coating process for at-line or in-line measurements. Combining this in-process measurement with a computational model, Colorcon’s My Dosage Design™, allows particle size and coating thickness to be measured in real-time and compared to a predictive model (reference: Colorcon AAPS Poster, 2017). This allows for early detection of processing issues (poor efficiency, agglomeration) with improved process control tracking and coating endpoint determination.


Considerations for Multiparticulate Processing
-
Computational design tools (My Dosage Design™) provide insight to important variables relating to a multiparticulate dosage formulation design and performance including particle size, surface area to film thickness (SA/FT) ratio, the volume of the final dose and optimal capsule size selection (reference: Colorcon CRS Poster, 2016).
-
PAT provides the basis for understanding relationships among multiple process parameters, formulation design and functional performance.
-
Real-time, at-line and in-line, process tracking of a fluid bed coating process provides enhanced understanding and control of product quality from development through commercialization and the lifecycle of a product.
Formulation and Process Knowledge to Speed Development and Build Quality
To assist with the development of multiparticulate formulations, Colorcon offers consulting through My Dosage Design™, a modeling tool that helps determine the optimal particle size, surface area to film thickness ratio, the volume of the final dosage and optimal capsule size selection.
Let us help to select your coating formulation and starting substrate for your next formulation. Connect with Colorcon to embark on a new multiparticulate project.