Formulation of Veterinary Dosages
Healthy Pets Make Happy People
It’s no secret our companion animals like to consume something that tastes good to them. While it’s clear pets have different tastes than us, they also avoid unpleasant-tasting medications. Highly palatable solid oral dosage forms that are freely accepted by the dog or cat, either from their feeding bowl or owners' hand, contribute to help the uptake of medicines. Great tasting solutions allow for easier dosing and improved compliance with the medication schedule. Pets are extensions of our families and deserve products that are safe, reliable, effective, and appealing.

Developing Veterinary Dosages
For veterinary medicinal products (VMP), convenience and compliance are critical to ensure the success of prevention, control, and treatment medication programs. With that in mind, manufacturers want to develop palatable solutions that offer a pleasant taste, smell, and mouthfeel that are voluntarily consumed and not ‘hidden’ in a more appealing food. As dosage forms for companion animals become more popular, companies producing VMP realize the value for ease of administration and compliance, especially for regularly given medications. However, tablets intended for veterinary administration may lack effective taste-masking of the drug substance or proper excipient selection, which can negatively impact palatability and voluntary acceptance.
Ingredients for Increasing Acceptance
Commercially manufactured VMP are often designed using ‘human’ formulation strategies. Although in many instances this works well, it doesn’t address the specific needs for a veterinary dosage form.
Colorcon’s proven and trusted Starch 1500®, partially pregelatinized maize starch, has been successfully used as a key excipient in formulating veterinary oral dosage forms.
As a naturally sourced excipient from identity-preserved non-GM corn and manufactured in dedicated GMP facilities, the use of Starch 1500 in veterinary dosage forms like tablets and soft chews provides unique benefits for manufacturers:
- Provides a neutral taste with good palatability - no chemical additives or surfactants are used in the manufacture of Starch 1500
- Exhibits good thermal stability to about 121°C, ideal for veterinary soft chew manufacture
- Imparts plasticity (ductility) to the dosage form and helps maintain these properties over the shelf-life of the product
- No animal products or by-products are used in the manufacture of Starch 1500
- Starch 1500 cuts process and material costs by reducing or eliminating binders, superdisintegrants, high levels of lubricants and glidants
Simplifying Your Formulation Strategy
Colorcon evaluated the use of Starch 1500 in a VMP to assess the suitability of use on tablet properties and performance. The study was conducted to demonstrate the value of a formulation strategy to reduce the number of excipients to a simple combination of partially pregelatinized maize starch (Starch 1500) and microcrystalline cellulose (MCC) in a tablet formulation (Table 1). The resulting formulation was then assessed using a range of different processing methods: direct compression (DC), top-spray granulation (TS), and high-shear granulation (HS).
By combining Starch 1500 with MCC in the tablet formulation the resulting tablets show good characteristics for manufacturing such as hardness, low ejection force, and friability. The tablets exhibit excellent disintegration and dissolution performance.
Table 1: Glucosamine Tablet Formulation
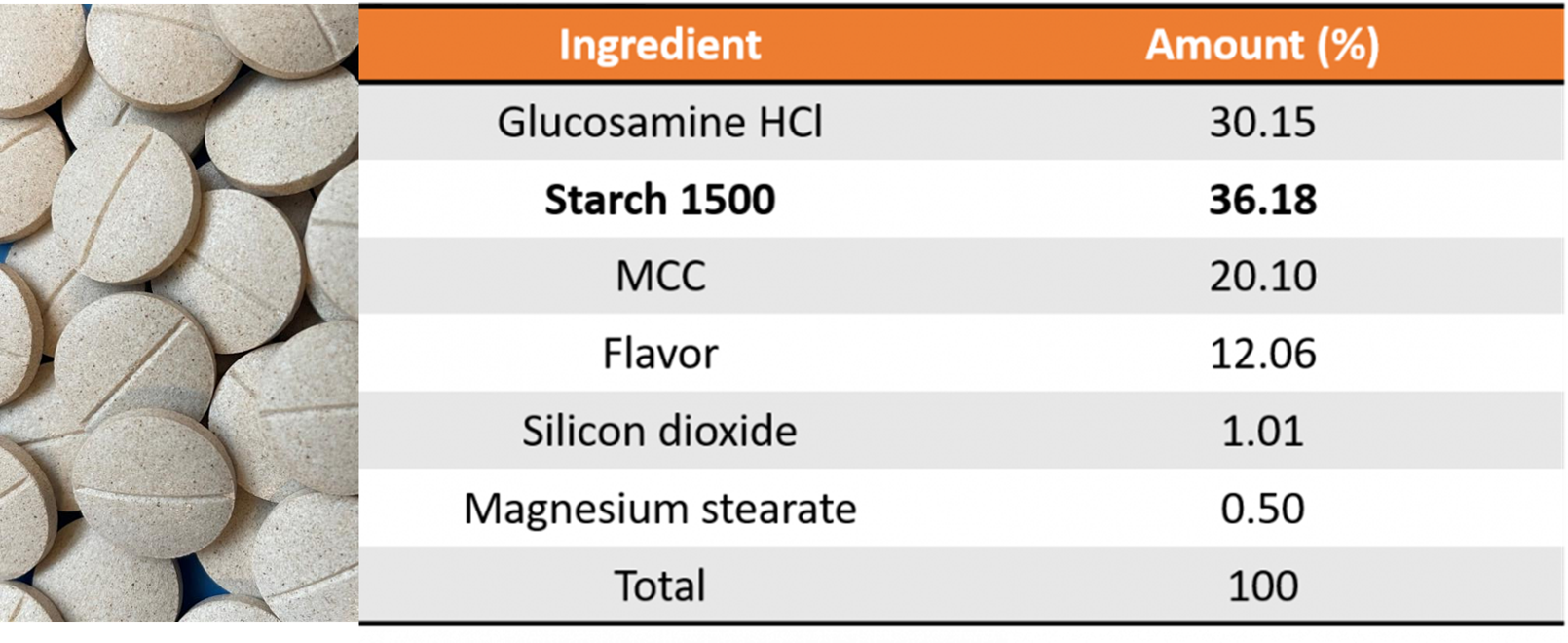
Finally, the binding properties of the Starch 1500, specifically when used in the wet granulation processes, enabled flowable granulations without the use of any polymer binders. This is a huge benefit for formulators when direct compression is not viable and granulation is the only option, specifically for low density, poorly flowing active pharmaceutical ingredients (API). Results concluded viable tablets were made using the different processing methods of direct compression (DC), top-spray granulation (TS), and high-shear granulation (HS), providing ease of formulation with manufacturing flexibility.
Overall, Starch 1500 provides formulation simplification and manufacturing efficiency for use in veterinary tablets; delivering a final product that will delight both pets and owners.
Colorcon provides high-quality functional excipients and fully formulated film coating systems that support taste-masking and palatability to expedite the development of VMP and deliver more palatable products that our companion animals deserve.

