

Design of Generic Tablets & Capsules
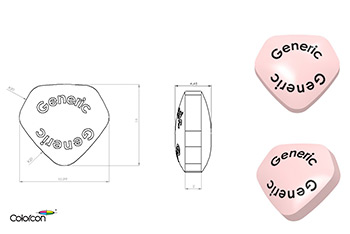
The US Center for Drug Evaluation and Research (CDER), part of the FDA, published its final version of a Guidance for Industry document: (2015) Size, Shape, and other Physical Attributes of Generic Tablets and Capsules applying to abbreviated new drug applications (ANDAs).
The guidance conveys the FDA’s current thinking in relation to the design of generic tablets and capsules; and how manufacturers should ensure the design of a generic version does not hinder patient compliance.
Colorcon is well positioned to support its customers in relation to the recommendations of this guidance.
- BEST®, unique tablet design service, explores tablet design options and can be used to evaluate tablet shapes and sizes for ease of swallowing
- Over 50 years’ experience in the development and manufacture of fully formulated coating systems, each optimized for color, finish, and specific application
More than Monograph – Suglets® Sugar Spheres
At Colorcon, we maintain unique quality standards for our sugar spheres, SUGLETS®, going beyond the pharmaceutical monograph test requirements. To ensure a highly consistent product, we focus on three Critical Quality Attributes (CQAs): sphericity, particle size and friability.
Friability presents a specific user concern. During the drug layering process it can result in changes to particle size and loss of sphericity, potentially resulting in variability in drug release. Since there are no monograph limits for friability, we set out to raise standards, and through more relevant testing, deliver Suglets to our customers with lower friability.
Oscillating friability is shown to be an appropriate method to determine robustness; and with two years of historical data, Colorcon has implemented internal friability limits to ensure a robust, reliable substrate for our customers, especially when using Suglets with fluid bed processing. Today, all shipped batches meet our new lower friability specification, giving our customers consistent, high-quality product and peace of mind. Read more

We have compelling data to share with you about consistency and control of all the CQAs that are important to your development and manufacturing. Contact your Colorcon Area Technical Manager for more detail, and to reduce your development time ask for a demo of our new My Dosage Design Calculator.
Taste-mask Powder Layering of Highly Soluble, Irregularly Shaped Drugs

Taste-masking can be particularly challenging for highly soluble or irregularly shaped drugs. In a Colorcon-Merck joint poster presented at the 2015 Annual AAPS Meeting, we set out to mask the taste of these challenging APIs and evaluate the use of ETHOCEL™ FP, fine particle ethylcellulose, in powder-layering applications. In rotor granulation, a rapidly rotating plate produces dense, spherical particles, and the high tangential forces work to coalesce the powder film in the presence of an aqueous binder. The low operating temperatures and quick application rates make powder layering a good option for the challenges of moisture and heat sensitive drugs. Read more

Keeping Pace with Continuous Coating
Colorcon continues to support its customers interest in the rapidly evolving continuous coater technology area. The July 2015 Solid Dose Newsletter featured our work evaluating tablet movement, transit times and coating uniformity in a high throughput continuous coating process. Since then, we have completed an evaluation of our novel, developmental, film coating formulation capable of application at solids concentrations as high as 35%. The study was conducted in DRIACONTI-T® continuous-cycled coater that employs a unique, segmented drum configuration to convey the tablets through the coating process. Our trials resulted in excellent coated tablet appearance at low coating weight gain with color consistency. Details of this study are published in the October 2015 issue of Tablets and Capsules Evaluation of a Continuous-Cycled Film Coating in Applying a High-Solids Coating Formulation.


Dosage Form Design
Since 2007, when the EU Pediatric Regulation (191/2006) came into force, pharmaceutical companies have been obliged to complete a Pediatric Implementation Plan (PIP) for their molecules. The objective of the regulation is to ensure that medicines for use in children are high quality, ethically researched, authorized appropriately and improve the availability of information on the use of medicines for children.

The EuPFI (European Pediatric Formulation Initiative), a collaboration of academia, clinicians and industry, has been instrumental in promoting the development of pediatric medicines. The Geriatric Medicines Society is aiming to do the same thing for geriatric dosages.
The key need for the pediatric population is to have access to safe, efficacious medicines; the requirement for formulators is to develop a dosage form that is age suitable, with good taste-masking, using appropriate excipients and is easy to administer. A risk-based approach to formulation decisions is a considered way to approach pediatric dosage form design and becomes the basis of the PIP. The trend that we see is towards solid dose formulations that enable dose manipulation (such as multiparticulates) rather than liquid formulations.
Geriatric or “not so young” populations were initially included in the scope of most pediatric medicine development departments, but it soon became clear that the needs of the two populations are very different. While they do share some common problems, such as swallowing difficulties, the geriatric population needs a dosage form they can handle easily as well. Colorcon’s BEST (Unique Tablet Design) specialists can help design tablets to improve handling and minimize gagging, with recommendations for a suitable film coating that does help the “medicine go down”.
Taste for geriatric patients is still a consideration but is not as critical to compliance as it is in the pediatric population. The physical design of the dosage form, easy to open packaging and methods to organize polypharmacy dose regimes, are key considerations for geriatric patients.
The path to achieving a pediatric regulation in Europe was long and required input from many sources. Geriatric medicines are heading down this pathway with the EMA Geriatric Medicines Strategy published in February, 2011. It will be an interesting journey, and with a growing, more affluent population suffering from a wider range of diseases, it is one that will be followed closely.
Opening Up Colorant Options for US Market
FDA’s approval of Colorcon’s Color Additive Petition (CAP 4C0300) expands the use of Spirulina Extract in coating formulations applied to dietary supplements, drug tablets and capsules marketed in the United States. Colorcon developed its petition in response to requests from dietary supplement manufacturers for more naturally derived pigment options, specifically stable blue colors, which are difficult to achieve. The FDA, after their review of Colorcon’s updated safety assessment and additional product stability testing agreed to expand the range of product categories used in coating formulations in which Spirulina Extract can be used to include all types of pharmaceutical and dietary supplement tablets and capsules in the United States. Colorcon has confirmed with FDA that this approval also covers the use of Spirulina Extract in printing ink formulations applied to dietary supplement and drug tablets and capsules since the print films that result on the surface of a tablet or capsule are essentially discontinuous coatings.
Incorporating pigments that are more “natural” requires specialized formulation and extensive stability programs to obtain the best quality results, including acceptable shelf‐life for the final coated tablets. At the time of the approval, Colorcon had stability tested and approved over 60 coating formulations containing Spirulina Extract added to its system, ready for immediate use. Read more


Shanghai Pharmaceutical Professional Association – Simon Tasker Scoops Leadership Award

Colorcon is extremely proud of Simon Tasker, Managing Director for his personal accomplishments and business achievements managing Colorcon operations in China. That’s why we were thrilled that the Shanghai Pharmaceutical Professional Association recently presented him with the February Flower Business Leadership Award. This prestigious award, given to business leaders of pharmaceutical and associated companies operating in Shanghai, recognizes Simon’s special commitment in leading and coaching his Colorcon team to success.
Colorcon is known as a leader in excipients across China, where concern over excipient sources, traceability and quality have grown in importance. Security of supply coupled with outstanding service and supported by Simon with his expert team, means Colorcon is recognized as setting the highest standard for excipient suppliers in China.
Of the ten winners, Simon was the only non-Chinese recipient, and Colorcon was the only excipient company, with representation in the winner’s circle. The awards were rewarded based on the personal stories, achievements and leadership styles.
Congratulations to Simon, and the entire China team, for an exceptional award.


